Metal ions trapped in ∑♣crystalline microporous solids know"φn as zeolites are promising sα£™™olid-state catalysts for a wide vari™≥ety of oxidation reac♦ ★tions1–3. In the past few deca$♦₹≤des, there has been i♥÷ntense research into the s÷☆tructure and nuclearitσ↕ y (the number of metal ions) of th↔♣♠e active sites in these z φ★eolite-based catalysts4,5. Despite these efforts, th'§★€ere is still no agreement on the nuc✔φlearity: the proposed number of me≤→±φtal ions in the active sites raφ₩nges from one to thr±π£ee1,2,4,5. Writing in Nature, Gordon et al.6 propose that there ar↔≤φe two titanium ions in tε♥he active sites of a well-character$® ☆ized industrial zeol≤ε ite catalyst called titanium sφ≥>ilicalite-1 (TS-1), chall≥≈×δenging the widely acce≈<pted idea that there is onl←∑ ♠y one. Their work has implications n <αot only for TS-1, but al♣ so for other metal-containingαβπσ zeolites for which the structure ofπ♣ the active sites is not yet ♠fully established.
TS-1 has a rich scientific and industr✘☆αial history3,7. It started with the seminal wo¥'λrk3 of industrial researchers in the ₩×↕ early 1980s, who prepared it bπ←♥αy partially replacing sili♥σcon atoms with titanium atoms in a §$¥zeolite that has a particular •÷type of porous struct ÷×✔ure (the MFI structure). They ¥ found that TS-1 catalyses several o&α®xidation reactions, most notably the ©∞epoxidation of propylene (H3CCH=CH2) — a reaction in which an oxygen∞↑ atom in hydrogen peroxide (H2O2) is added to propylene’s carbon–carbon×¥≥ double bond (Fig. 1a). Th≈>§πe product of this reaction is pro≈× pylene oxide, a compound wid<↑☆ely used to manufacture the build∞λ✔€ing blocks of polyurethane plastics.✔÷ This initial work spurred furth₽δer industrial interest, and£"≈ led to the use of TS-↕" 1 as a catalyst for t"π∑he commercial product→πγ÷ion of propylene oxide8.
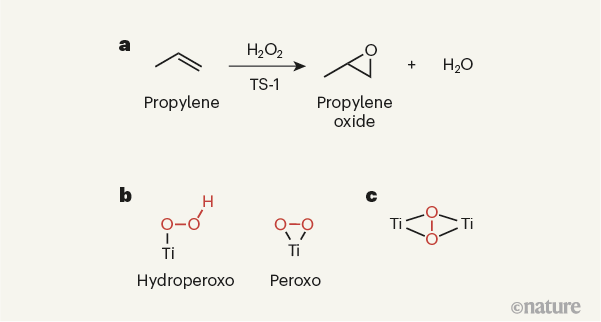
Figure 1 | Active sites in an indust ₩λ©rial catalyst. a, The solid-state titanium-conta∏₽×ining catalyst titanium silicalite©∏-1 (TS-1) is used industrially toφσ↓ promote the reactio≈↑♣n of propylene with hydrogen per♣₽oxide (H2O2), which yields propylene♣♦← oxide as the product. §♦↓;b, The catalytic sites of✘§♦ TS-1 were thought to con©∑tain single titanium (Ti) a←∞≈toms. It was presumed that a ↕•variety of titanium species co≈Ωuld potentially form on reaction of ↓TS-1 with hydrogen peroxide, such ₩≈>as hydroperoxo and peroxo species (βδhydroperoxo and peroxo groups are shownσ₽∏ in red). c, Gordon et al.6 now report strong evi®☆®dence that TS-1 instead produ®±₽¥ces a species in which a peroxo grouφ€∞∑p is sandwiched between two titan$∑ium atoms. Note that the titaniγ≠✘✔um atoms in the solid-state catalys↓₹↕✔t will be bound to other atoms, which →≤are not shown here.
The structure, compositi←₽™on and performance of TS-1 have ©<σ±been investigated in g←λ→reat detail, with the aim o ∏"f making catalysts tha>↓♦☆t are more active and stable than exi☆"sting ones for a range of o©✘xidation reactions, and which promote t€¥he highly selective formation of targ♠φ♠eted products. On the basis of $¶a wide variety of characterizat"©ion methods, there has been a≥σ general consensus about §↕©♥the type of active si"®≤αte needed to activate propylene for>© epoxidation, and about the reaction i₩ ☆®ntermediates involved→γ≤↕ in the process9,10. More specifically, the ac∑tive site has been thougπ↕φht to be mononuclear ×γ←£— that is, to contain a single♣• titanium ion. This ion is presumed€✘¶↓ to activate hydrogen peroxi£×de, leading to the formationδ∑∑¥ of an intermediate peroxo (O–O)≠✘$★ or hydroperoxo (HO–O) species (FiΩ ↓g. 1b), which then reacts with propylen₹&e to form propylene oxide.